Abstract
Introduction:
While BCR-ABL1 negative myeloproliferative neoplasms (MPN) are typically diagnosed in the sixth decade of life, approximately 20% of patients are diagnosed before the age of 40 years. Patients with MPN of reproductive age are being increasingly encountered in clinical practice. Concurrently, there has been increased awareness of the risks of pregnancy complications in patients with MPN and the importance of MPN-specific management to help mitigate these risks. MPN are associated with thrombotic and hemorrhagic complications, and pregnancy may amplify the thrombotic risk. Additionally, MPN may be associated with an increased risk of placental dysfunction and associated complications of preeclampsia, fetal growth restriction, preterm delivery and fetal loss. The aim of this observational study was to report on pregnancy outcomes in a modern cohort of patients with MPN managed according to consensus recommendations.
Methods:
We conducted a retrospective review of patients with MPN and pregnancy evaluated at either the Princess Margaret Cancer Centre or Mount Sinai Hospital in Toronto, Canada between January 1, 2010 and December 31, 2020. Diagnoses were defined according to the WHO 2016 criteria using information available from hospital records. Descriptive statistics were used to describe selected baseline characteristics. Categorical variables were summarized with counts and percentages.
Results:
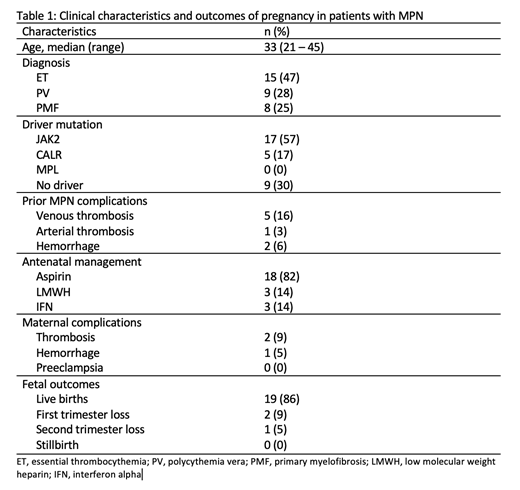
A total of 32 patients with MPN and pregnancy were included in the study (Table 1). The median age at the time of the index pregnancy was 33 (range 21 - 45) years. The most common MPN diagnosis was essential thrombocythemia (ET, n=15), followed by polycythemia vera (PV, n=9) and primary myelofibrosis (PMF, n=8). Driver mutation data was available for 30 patients: 17 (57%) had mutated JAK2, 5 (17%) CALR, and 9 (30%) had no driver mutation identified. Five patients had a prior history of venous thrombosis, all of which were portal vein thrombosis, and 2 patients had a history of bleeding events. Information on antenatal treatment was available for 22 patients: 18 (82%) patients received aspirin, 3 (14%) received antenatal low molecular weight heparin (LMWH) and 3 (14%) received interferon (IFN; interferon alpha 2b in 2 cases and pegylated interferon alfa 2a in 1); 16 (73%) received post-partum LMWH.
Information on maternal complications was available for 22 patients. There were 2 thrombotic events (1 antepartum and 1 postpartum) and 1 postpartum hemorrhage. There were no cases of preeclampsia. Of 22 pregnancies, there were 19 live births (86%), 2 first trimester losses (9%) and 1 second trimester loss (5%). Gestational age was 37 weeks or more in 16/17 (94%) and was 33 weeks for 1 patient. Vaginal deliveries were performed in 11/20 (55%) cases and 9/20 (45%) were Cesarean deliveries.
Discussion:
This observational study represents a modern cohort of MPN patients treated according to consensus recommendations. Our findings highlight that MPN patients have better pregnancy outcomes than those previously described in the literature. Limitations of this retrospective study include a small sample, missing data, and potential underreporting of early pregnancy loss. Pregnancy in patients with MPN is associated with unique risks that may be reduced with interventions such as antepartum aspirin, IFN in higher risk patients, and postpartum LMWH. That not all standard risk patients were managed with aspirin and postpartum LMWH suggests educational opportunities exist for hematologists and maternal-fetal medicine physicians involved in the care of these patients.
Gupta: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria; Pfizer: Consultancy; Roche: Consultancy; Constellation Pharma: Consultancy, Honoraria; Sierra Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Research Funding. Malinowski: Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy. Maze: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria; Takeda: Research Funding; PharmaEssentia: Research Funding; Kronos Bio: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal